top of page

Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge whereas the electrons have a negative electric charge. The neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral.
the atomic structure
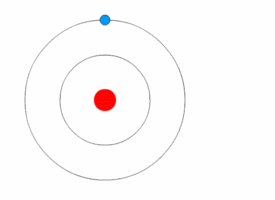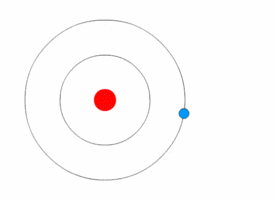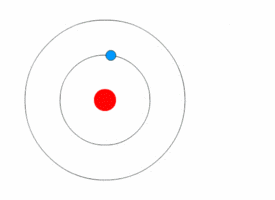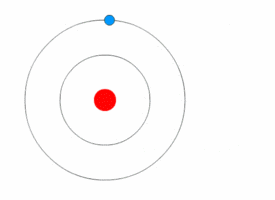
The Excited Atom
It is an atom that gain quantum of energy
After absorbing energy, an electron may jump from the ground state to a higher energy excited state.

bottom of page



